The FDA recently issued a Covid-19 vaccine warning myocarditis update, highlighting new safety information for mRNA vaccines like Comirnaty and Spikevax. This update is devoted to the uncommon threat of myocarditis and pericarditis, especially in younger men following vaccination.
Both healthcare providers and the public should be aware of these developments to make safe vaccination choices and identify the possible side effects early. Knowing this new information will assist people to balance the advantages and disadvantages correctly and encourage people to have confidence in COVID-19 vaccination programs.
What Is Myocarditis and Pericarditis?
Inflammation of heart muscle is called myocarditis and inflammation of the protective lining of the heart is called pericarditis. The two may affect the functioning of the heart and happen as a result of infection, immune reaction or, infrequently, as a vaccination side effect. The symptoms usually involve chest pain, dyspnea, weakness, and palpitations. The majority of cases are mild and heal with treatment although few may have long-term effects that need constant medical attention.
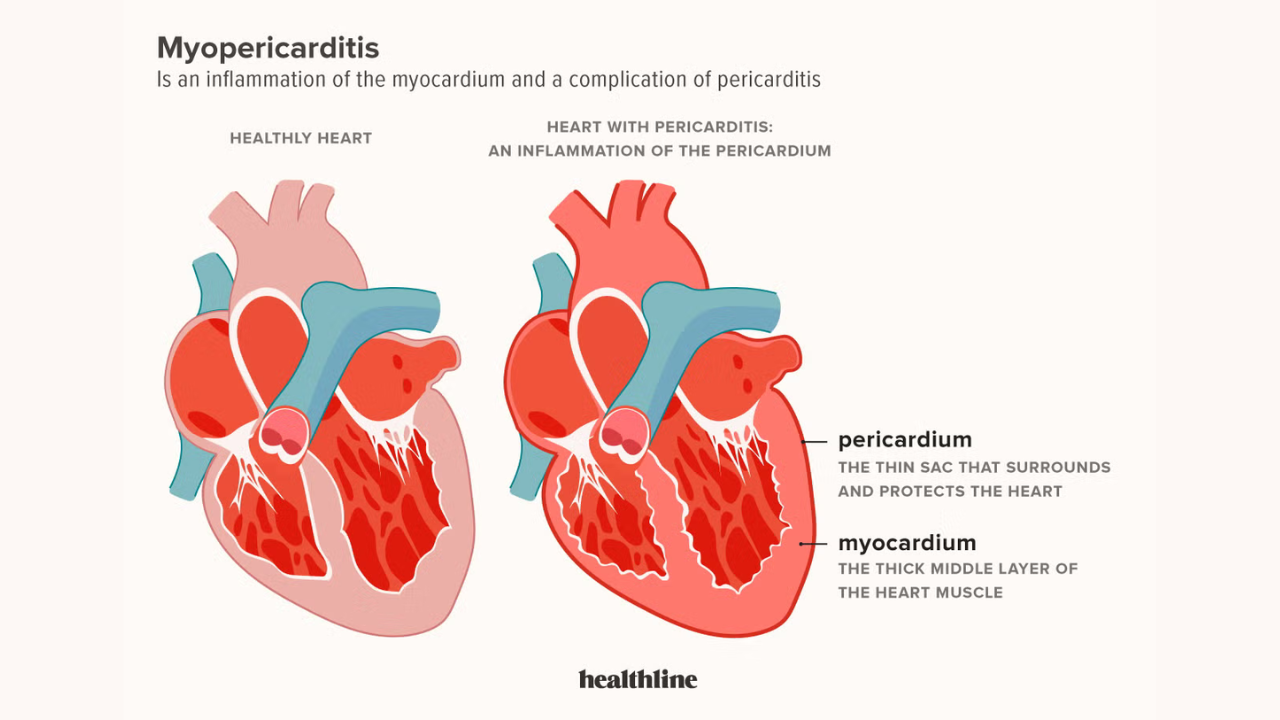
The FDA has recently issued a Covid-19 vaccine warning myocarditis update, highlighting that these side effects, though uncommon, are more frequently observed in younger males after mRNA COVID-19 vaccination. Early intervention and improved outcomes of the affected individuals are guaranteed by understanding the signs and immediate medical attention.
FDA’s Updated Warning on mRNA COVID-19 Vaccines
On June 25, 2025, the FDA required changes to the prescribing information of mRNA COVID-19 vaccines- Comirnaty (Pfizer-BioNTech) and Spikevax (Moderna) to include more warnings regarding the risks of myocarditis and pericarditis. These are updated according to new data of the 20232024 vaccine formulations and results of a longitudinal study of cardiac magnetic resonance imaging (CMR) in people who had developed myocarditis after vaccination.
Key updates include:
Estimated Incidence Rates: The unadjusted myocarditis and/or pericarditis incidence of 1-7 days after vaccination is estimated at 8 cases per million doses in 6 months to 64 years of age. The incidence increases to about 27 cases per million doses in males between the ages of 12 and 24 years.
Long-Term Cardiac Results: A cohort study of nearly 300 people who experienced myocarditis following vaccination found that a large proportion of them had sustained abnormal CMR results at a median of approximately 5 months. The clinical implications of such results are under study.
New Safety Data: The Fact Sheets of Healthcare Providers and Recipients and Caregivers are updated with these new safety data, so that the medical community and the general population are notified about the potential risks of these vaccines.
These updates highlight the FDA in its efforts to present clear and evidence-based information to inform the decision-making process in terms of COVID-19 vaccinations.
Incidence Rates and Risk Assessment
The FDA’s Covid-19 vaccine warning myocarditis update highlights the estimated incidence of myocarditis and pericarditis following mRNA COVID-19 vaccinations. The unadjusted incidence was about 8 cases per million doses among persons aged 6 months to 64 years during the period 1-7 days following vaccination with the 20232024 mRNA vaccine preparations. In males aged between 12-24 years, it rose to about 27 cases per million doses.
In comparison, according to the data of 40 U.S. healthcare systems, the risk of myocarditis is much greater after a COVID-19 infection than after vaccination. As an example, males aged 1217 years were at 1.856 times increased risk of myocarditis following a COVID-19 infection than following a second dose of mRNA vaccines.

These results highlight the need to learn the comparative risks and benefits of vaccination against infection.
Post-Vaccination Monitoring and Outcomes
Post-vaccination follow-up studies of patients who developed myocarditis following mRNA COVID-19 vaccination show that the majority of patients have positive clinical outcomes. A systematic review of 27 studies showed that symptoms had resolved in all patients, but 18.7% had abnormal electrocardiography and 3.8% had high levels of troponin at follow-up.
Cardiac magnetic resonance imaging (CMR) at a median of 3 to 6.3 months after vaccination indicated that 76 percent of patients had persistent late gadolinium enhancement (LGE) but with less intensity than during the acute phase. The average left ventricular ejection fraction increased by about 3 percent as compared to baseline.
Also, a longitudinal study in Denmark of 17 vaccine-associated myocarditis patients reported mild left ventricular dysfunction and diastolic abnormalities in 71.4% of patients. Although biomarkers were normalized, 35 percent of patients experienced symptoms that were not cleared (fatigue and chest pain), which highlights the importance of continued monitoring.
The results indicate the necessity of further monitoring and personalized treatment of patients with myocarditis after vaccination.
Guidance for Healthcare Providers
Healthcare providers ought to be careful to observe patients, especially males aged 1224 years, to identify myocarditis or pericarditis after receiving mRNA COVID-19 vaccinations. The symptoms usually appear within one week after the vaccination and can be chest pain, dyspnea, or palpitations.
Recommendations:
Early Detection: Early assessment of patients with the presenting symptoms of chest pain or related symptoms after vaccination.
Diagnostic Testing: Use electrocardiography (ECG), cardiac biomarkers (e.g., troponin), and echocardiography to determine cardiac involvement.
Reporting: To facilitate continuous safety monitoring, report suspected cases to the Vaccine Adverse Event Reporting System (VAERS).

Treatment: A majority of the cases are treated conservatively through rest and anti-inflammatory drugs. Nevertheless, others might need to be hospitalized or treated in a special way.
To be more specific, consult the clinical considerations of myocarditis and pericarditis following COVID-19 vaccination at the CDC.
Read Also: Polynucleotide Treatment: Natural Skin Rejuvenation Without Fillers
Advice for Recipients and Caregivers
The revised Fact Sheets of the FDA on Recipients and Caregivers also contain more information about the infrequent risk of myocarditis and pericarditis after mRNA COVID-19 vaccines. Key points to consider:
Risk Awareness: The risk is low but higher in males aged 1224 years, with 27 cases per million doses in 17 days of the LVC, and 13 cases in 7 days of the LVC.
Symptom Monitoring: Pay attention to the appearance of such symptoms as chest pain, dyspnea, or palpitations, particularly in the first week after vaccination.
Immediate Action: Please visit a doctor as soon as there are any worrisome symptoms.
Informed Decision-Making: Talk to your health provider about any issues to make an informed decision on vaccination.
To find out more, see the FDA Fact Sheet on Recipients and Caregivers.
Final Word
The FDA’s Covid-19 vaccine warning myocarditis update emphasizes the rare risk of myocarditis and pericarditis, particularly in younger males following mRNA COVID-19 vaccination. Though the majority of cases are mild and can be treated, awareness and early diagnosis are of the utmost essence. Patients need to watch out for such symptoms as chest pain, dyspnea, or palpitations. The visit to a healthcare provider will guarantee individual advice and knowledgeable choices, allowing to weigh the advantages of the vaccination against possible dangers. Remaining educated helps in making safe and assured vaccination decisions.
Read More: 33 Proposal set for implementation for Bangladesh’s healthcare reform